A Surgeon Scientist, Physician, and Innovator
As an active researcher and surgeon scientist, Dr. Tewari is one of only a few robotic surgeons to be awarded NIH R01 and Department of Defense(DOD) Cancer Research grants. This funding supports the investigation of real-time tissue identification during prostate cancer surgery and immunotherapy.
Dr. Tewari also serves as site-PI for the NIH/NCI Cancer Genome Atlas Tissue Source Site and the NIH/NCI Early Detection Research Network. In addition to his federal funding, Dr. Tewari’s research is currently funded by the Prostate Cancer Foundation, Boston Scientific, and Medtronic. He has initiated collaborative work with many other leading institutions, including Cold Spring Harbor Laboratory, the Broad Institute of MIT and Harvard, the Koch Institute for Integrative Cancer Research at MIT, Dana-Farber Cancer Institute, and The Rockefeller University.

The Tewari Lab
As a researcher, Dr. Tewari has authored over 250 peer-reviewed articles, book chapters, and textbooks on prostate cancer and robotic surgery. His has published several high-impact articles in Nature, Cell, and PNAS.
The Tewari Lab is actively involved in the following areas of research: single-cell genomics; high-resolution MRI; development and evaluation of prostate cancer vaccines; techniques to improve nerve sparing and maximizing early return of continence and sexual function; advancement of MRI targeted biopsies and focal therapy; and novel intra-operative imaging techniques.
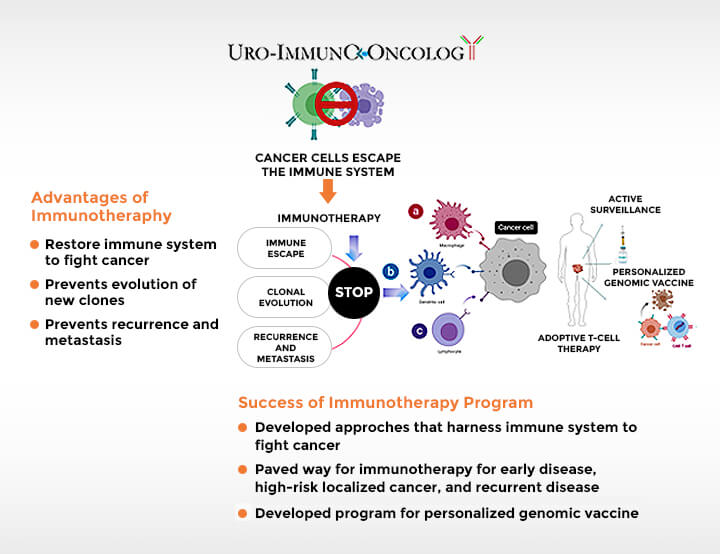
Dr. Tewari’s research approaches the problem of prostate cancer from distinct perspectives.
The Tewari Lab is working on uncovering the genomic causes of prostate cancer and translating genomic information to practical clinical application through imaging.
The Lab’s Outcomes Research Group is pursuing several research topics:
- Investigation of the epidemiological effects of prostate cancer
- Investigation of novel imaging techniques for identifying prostate cancer imaging biomarkers
- Development of effective immunotherapy agents, personalized to a patient’s own tumor and with increased ability to educate a patient’s immune system to eliminate cancer
Clinical trials bring life extending and curative new treatments to cancer patients. Clinical drug trials play a vital role in moving new treatments to patients who need them most, securing data so regulatory approvals can be obtained and new drugs can move into widespread clinical practice. Patients who participate in clinical trials provide an invaluable service both to treatment science and fellow patients.
Under Dr. Tewari’s leadership, novel and innovative trials are continually opening in the Department of Urology. This section will be updated frequently to reflect Work-in-Progress and ongoing trials in the Department. Please contact Dr. Tewari’s office to inquire about eligibility for trial recruitment. Here is a list of key clinical trials underway by Dr. Tewari’s team:
Neoadjuvant Hiltonol® (PolyICLC) for Prostate Cancer
The purpose of this study is to test an approach of stimulating the body's immune system to attack prostate cancer.
Upright MRI for Prostate Cancer Screening
This is an investigator-initiated study to test the efficacy of an upright MRI (Magnetic Resonance Imaging) for the screening of prostate cancer. The purpose of this study is to compare Upright MRI as a technique to PSA (Prostate Specific Antigen) and current MRI imaging.
Feasibility of the LUM Imaging System for Detection of Prostate Cancer
The primary objective of this feasibility study is to determine if administration of LUM015 will result in positive fluorescence of tumor tissue from ex vivo specimen imaging with the LUM Imaging device from patients undergoing radical prostatectomy for prostate cancer. Both normal tissue and tumor tissue will be imaged and analyzed. The LUM Imaging System is a portable combination product consisting of an imaging device and an imaging agent (LUM015).
Safety and Efficacy of MAGE-A3/A6 T Cell Receptor Engineered T Cells (KITE-718) in HLA-DPB1*04:01 Positive Adults With Advanced Cancers
The primary objectives of Phase 1A are to evaluate the safety of KITE-718, determine a recommended Phase 1B dose, and to evaluate the efficacy of KITE-718 in Phase 1B.
EDRN Prostate MRI Biomarker Study (P-MRI)
The commercialization of MRI fusion biopsies has resulted in a dramatic increase in the use of MRI imaging for prostate cancer. How best to use MRI in the initial prostate biopsy setting given the availability of validated prostate cancer early detection markers is uncertain. This study will allow investigators to determine if prostate MRI is superior to validated panel of laboratory biomarkers (e.g. PCA3, PSA and TMPRSS2:ERG) in the initial biopsy setting.
HRQol (Health related quality of life)
An observational study to learn about the natural course of prostate and bladder cancer. To investigate genetic changes associated with prostate cancer, bladder cancer and other diseases.
BED PSMA-301 - LIGHTHOUSE Study
Phase III imaging study investigating a radiohybrid prostate-specific membrane antigen (rhPSMA)-7.3 (18F) PET ligand for detection of N1 and M1 metastases.
BED PSMA-302 - LIGHTHOUSE Study
Phase III imaging study investigating a radiohybrid prostate-specific membrane antigen (rhPSMA)-7.3 (18F) PET ligand in men with suspected PCa recurrence based on elevated PSA following prior therapy.
Cancer Biorepository
Collecting samples of tissues from patients undergoing surgery for a tumor, for scientific studies to better understand the tumor biology.
IntraTumor Heterogeneity
Understanding solid tumors heterogeneity through comparative analysis from biopsies (kidney, prostate).
Kite-718
The study will evaluate the safety and preliminary effectiveness of an investigational immunotherapy called KITE-718. This treatment is comprised of immune cells called T cells which have been removed from the patient, modified in the laboratory to recognize a protein called MAGE-A3/A6, multiplied, and then returned to the patient to find and kill cancer cells.
LUMICELL
The study will evaluate the safety and preliminary effectiveness of an investigational immunotherapy called KITE-718. This treatment is comprised of immune cells called T cells which have been removed from the patient, modified in the laboratory to recognize a protein called MAGE-A3/A6, multiplied, and then returned to the patient to find and kill cancer cells.
PRO-VENT DENDREON
A Phase III, Active surveillance, an autologous cellular immunotherapy administered to patients on AS.
Continuous Database of Genitourinary Cancer
Giving permission to use health information to build a clinical database.
Artificial intelligence and neural networks in urology
Development of a model to predict prostate cancer at the apex (PCAP model) in patients undergoing robot-assisted radical prostatectomy
Novel nomogram for the prediction of seminal vesicle invasion including multiparametric magnetic resonance imaging

 International Patients
International Patients